Also In This Issue
Hope for earlier dx of breast-cancer recurrence
Predicting AML recurrence after stem-cell transplant
Bird flu update: Another person and more species
Lawsuit challenging FDA final rule on LDTs
New and Noteworthy
Towards a truly portable MRI? AI is making it possible
The technology of today’s MRI appears truly magical (AC Clark’s 1968 3rd law). The science behind it is based on Einstein’s Nobel prize-winning 1905 paper on the photoelectric effect, which later led to quantum mechanics. Tech-wise, its predecessors are the NMR machine (late 1930s) and the first clinical MRI machine in the 1980s.
Modern-day MRIs require incredibly strong magnetic fields. The problem is that the strength of a magnetic field declines with the cube of the distance from the field’s source. So imaging a two-foot-wide human requires a magnet that’s ~6,000x stronger than the one you’d need to image a two-inch-wide mouse. That’s why early commercial machines only imaged human heads (and research mice).
Most of the cost of today’s MRI machines ($1 million-plus) pays for a super-powerful superconducting liquid-helium magnet. The pulsing magnets perturb water in tissues, which generates the very weak radio signals used to create MRI images. Typical MRIs at large hospitals and imaging centers use a 3- to 5-Tesla magnet. (Tesla is the unit of magnetic field intensity. Three Tesla is about 3,000x the strength of your average magnet on your fridge).
Using such a big magnet increases the machine’s Signal (the image you want) to Noise (everything else) Ratio (SNR). It also increases the speed at which you can capture an image. That’s crucial not just because it’s no fun to be trapped in a noisy, claustrophobic tube for a long time but because it determines what you can see. If it takes half an hour to generate an image with a given MRI, you can’t use that machine to scan a heart that beats 60 times a minute.
Once you’ve got your (multi) million-dollar magnet, you need another $500K to $1 million to build a facility that can deliver 80 kilowatts of peak power (which also costs money). That facility must also have a magnetic-free zone around your MRI machine, to avoid catastrophic attraction of any iron-containing objects to the giant magnet.
That’s where we are now. The ultimate goal for MRI technology is a machine that can be plugged into a wall outlet and uses small, permanent magnets to deliver a clinically effective image (with high SNR) in 30 minutes or less. It would be safe for those with implants, require no special screening or facilities construction, and be small and cheap enough for the bedside and/or a (larger) physician’s office.
This month, Science reported progress toward that goal (see also this 2021 paper from the same group of scientists). AI is the key ingredient. With AI, a small, 0.05 Tesla machine, despite its lower SNRs, was able to generate a usable image.
Commentary: Different clinical applications have different MRI requirements, and meeting all of these requires very specific algorithms and methods. All the major commercial vendors (e.g., GE, Philips, Siemens) are working to downsize their MRIs to broaden the facilities that can use them, reduce cost, and increase patient access. It is all up to practical engineering solutions now. The clinical value of inexpensive and radiation-free soft-tissue imaging is enormous - and it’s within our grasp.
ctDNA testing may improve early detection of breast cancer recurrence
For breast cancer, recurrence testing is essential. Unfortunately, recurring tumors are typically recognized only after they have grown to 1-2 cm, the size at which they become visible on imaging. (In theory imaging should be able to catch much smaller tumors, but in practice they’re easily obscured by image noise).
This week, several researchers at the 2024 American Society of Clinical Oncologists (ASCO) conference presented smallish but very promising trials of commercially available tests that seem to be able to detect recurrent breast cancer at a much earlier point. The tests look for circulating tumor DNA (ctDNA).
One test was widely reported to promise “100% accuracy” at identifying recurrence an average of 12.5 months before the development of symptoms or the discovery of a tumor on imaging. The study behind the claim was small. It involved just 76 women, of whom the test identified all 10 who had relapsed (hence the 100% claim). But based on that data, it’s the most sensitive test available, identifying up to 1,800 unique mutations from each patient’s own tumor.
Commentary: More validation is clearly needed, but the news is heartening. That said, catching tumors early isn’t going to completely solve the challenge of breast-cancer recurrence. There is surprisingly little hard evidence (yet) that early detection results in improved outcomes in practice. That’s in part because we don’t (yet) have effective treatments for these recurrent tumors, which tend to be more aggressive - even if discovered early. While that is sobering news, the ability to detect recurrence early is critical to the ability to research and create treatments to attack the disease.
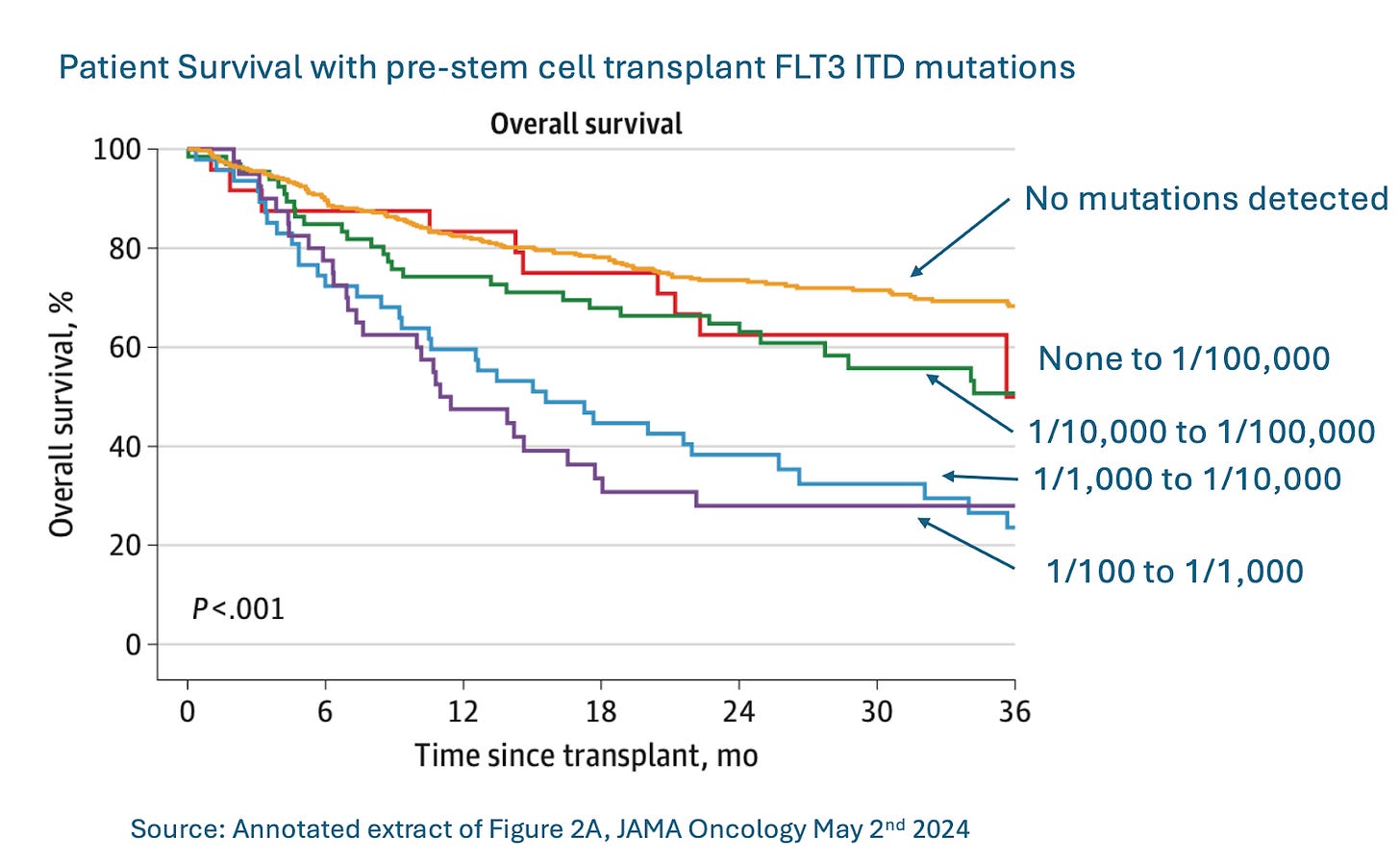
Test to predict leukemia recurrence after stem-cell transplant
A stem-cell transplant is one of the most arduous, complex and challenging medical procedures there is, but for some cancers it’s potentially curative. For one type of cancer, at least, clinicians may now be able to find out in advance whether that treatment is likely to be successful for their patient.
A recent JAMA Oncology paper demonstrates that for patients with acute myeloid leukemia (AML), if even a single mutant cancer cell (FLT3-ITD) in 10,000 persists after initial therapy, stem-cell transplants are much less effective at eliminating recurrence. The lower the number of these mutant cells was, the more likely it was that stem-cell transplant would work. This approach is called minimal residual disease (MRD) testing. It’s specifically designed to look for small numbers of cancer cells.
Commentary: This study provides a great example of how important it can be to identify the relevant biomarker and then to test for MRD both during and after treatment. These tests can help physicians make the best treatment choices for cancer patients before waiting for disease to recur.
Bird flu update: Another person, plus alpacas and house mice
A third person has tested positive for H5N1 - a dairy worker in Michigan, the state that’s been hardest hit by the disease in cows. This person had mild respiratory symptoms along with the conjunctivitis that was seen in the previous two cases, but no human-to-human transmission has been detected.
Despite additional incentives, individual testing of both people and cows continues to lag. The USDA has launched a program that allows herds which have tested negative three weeks in a row to participate in voluntary weekly bulk milk testing. Producers whose herds test negative on bulk milk samples will then be able to transport their cattle across state lines without further individual testing.
Dairy cows in Iowa and Minnesota have tested positive for H5N1, bringing the total number of (officially) affected states to 11.
The strain of H5N1 that affects cows has spilled into other species, including birds, cats, and alpacas. This suggests that while the disease is mainly being spread among cows via milk, it may also be transmissible in other ways.
H5N1 has also been detected in house mice - a concerning development because that species lives right where humans do.
The LDT lawsuit we knew was coming
The American Clinical Laboratory Association (ACLA) has filed a lawsuit challenging the FDA’s right to regulate laboratory-developed tests (LDTs) as medical devices. The ACLA argues that LDTs aren’t devices but services, which are regulated differently, and that the new rule would put too great a burden on labs.
COVID EUA Update
The FDA issued no new COVID 510(k) premarket notifications, no new EUAs, seven amendments existing EUAs, one new revocation, and one warning letter in May.
510(k) Premarket Notifications: 0
New EUAs: 0
Amendments to Existing EUAs: 7
Molecular: 1
COVID Antigen: 6
Revocations (1): Accula SARS-Cov-2 Test
Warning Letters: 1 (Cue Health) [Press]