ALSO IN THIS ISSUE
HHS Layoffs
Can a smell test diagnose the earliest stage of cognitive impairment?
A wearable computer?
Colonoscopy vs. FIT: Acceptance and efficacy
First Fully At-Home STI Test Approved
Court Vacates FDA’s Final Rule on LDTs
One of the two biggest diagnostics stories of the week is the decision by Judge Sean D. Jordan of the US District Court for the Eastern District of Texas to vacate the FDA’s Final Rule on laboratory-developed tests (LDTs). As a result, the compliance procedures and associated deadlines outlined in the Rule are no longer in effect. The decision centered on two issues.
According to the court, a laboratory-developed test is a service, not a device. If we were to use the FDA’s definition of devices, Judge Jordan wrote in his opinion, the agency would have regulatory oversight of all surgical procedures and medical examinations.
“Congress has considered but declined to enact several bills over the past two decades that would have reshaped the regulatory framework” around LDTs, the court noted. Thus, the court interpreted FDA’s Final Rule as an attempt to circumvent Congress’s authority.
COMMENTARY: This decision will no doubt make the business of labs and diagnostics companies easier in the short term. Given the current headwinds, this decision is good news.
In the long term, however, we believe that this will hurt the industry. Without the rule, diagnostics will remain in a different category from other medical devices and drugs, because not all diagnostics will have FDA approval. And without the LDT Rule’s scheduled quality processes and the FDA’s official stamp of approval, many will continue to doubt the accuracy of lab-developed tests. We also believe that payers will continue to debate the use and appropriate reimbursement of these tests.
The FDA has 60 days to appeal, but we believe it is highly unlikely that the current administration will do so. And even if they did - and won - the agency would have nowhere near enough staff to implement the Rule. So it is unlikely that LDT regulation will resurface anytime soon.
We hope that labs and diagnostic companies voluntarily review their internal processes and implement those parts of the LDT Rule’s quality systems that they can incorporate into their processes without undue burden.
HHS Mass Reduction in Force
The other important story is the layoffs at the US Department of Health and Human Services.
Commentary: These workforce reductions - 10,000 people are being fired, while an additional 10,000 are taking early retirements or separation packages - are happening in the name of efficiency. Is there a need to be more efficient and effective - absolutely yes. But from what is public, no logic has been described to explain what was eliminated and why. The lack of explanation and plan going forward leaves us to believe that HHS has become an agency in which fealty is more important than the agency’s prime directive of protecting our health, as Dr. Peter Marks wrote in his well-publicized letter of resignation from the FDA, it is an agency where “truth and transparency are not desired”.
Here’s the list of eliminated sections of the agency, compiled by Your Local Epidemiologist. This list includes some of the most impactful and successful programs of the last half century.
“HIV prevention? Gone.
Asthma and air quality team? Gone.
Environmental hazard response? Gone.
Gun violence prevention? Gutted.
Communications? Gutted.
Worker safety? Gone.
Reproductive health? Gone.
Birth defects? Gone.
Disability health? Gone.
TB prevention? Gone.
Blood disorder programs? Gone.
National survey on drug use and mental health? Gone.
Lead poisoning prevention? Gone.
Water safety? Gone.
Tobacco control division? Gone.”
What does it all mean for diagnostics? The industry has fought for innovation and while the LDT Rule is gone, we still need HHS (especially CDC, CMS, FDA, NIH) and without adequate staff, the pace of innovation will likely slow. We hope that funding for diagnostics is not lost as we know that without an accurate and timely diagnosis, treatment - the most expensive part of healthcare, will definitely not be efficient or effective.
Today, our hearts ache for all who have lost their jobs. Tomorrow - we worry about all of us whose health and safety is at risk, especially those groups that seem to have been singled out for abandonment.
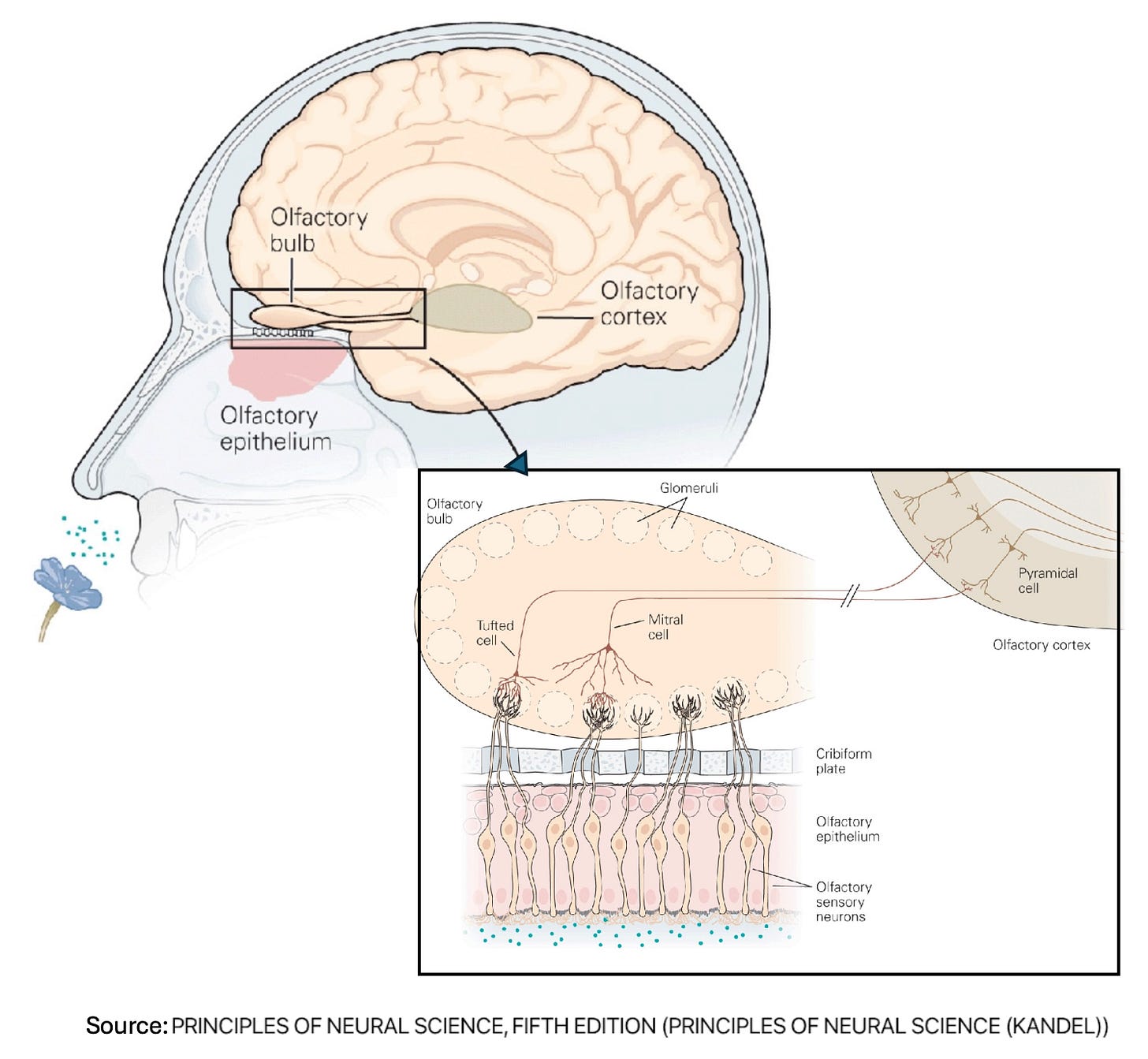
Does a smell test for the earliest stage of cognitive impairment pass the smell test?
Smell is a highly complex brain process. A limited number of odor receptors are closely integrated with several brain regions (the cortex, amygdala, and hippocampus) that are also essential to cognition. So could changes in one’s sense of smell be an early harbinger of more global cognitive decline?
A recent Nature/Scientific Reports article describes a simple at-home scratch-and-sniff test called AROMHA that was developed for this purpose. The test evaluates three capabilities: odor identification, discrimination between similar odors, and odor memory. While these capabilities do decline with age, this work provides evidence that cognitive and odor capabilities decline in parallel, beyond normal aging. The next step, the authors write, is to evaluate whether this test can also be used as a predictor of cognitive decline.
Measles Outbreaks Continue
The number of measles cases in the US this year just passed 500 - the largest yearly number since 2019, and it’s only the end of March. The current outbreaks are shown below. The cases in Texas, New Mexico, Kansas, and Oklahoma all came from the same source; the outbreak in Ohio is unrelated.
A whole computer in an elastic fiber? Now THAT’s a wearable
A couple of weeks ago, we reported on diagnostic pajamas that can monitor your sleep. Turns out that’s just the start. Researchers have created an entire computer - including sensors, a microcontroller, digital memory, Bluetooth modules, optical communications, and a battery that lasts for six hours - in the form of an elastic fiber that can be woven into machine-washable clothing. The whole thing weighs under 5g, and multiple computers can be networked together within a single garment.
The end result: Clothing that can monitor health and activity from locations closer to vital organs than a smartwatch or smart ring can reach. US Army and Navy service members will be taking the technology for a test run during an upcoming mission. The computers will be woven into the base-layer merino mesh shirts they’ll be wearing during a month-long stint in the Arctic.
Continuing on this theme - the littlest potential users may soon be able to benefit from wearable technology. Researchers have validated MAIJU (Motor Assessment of Infants with a Jumpsuit), an AI-enabled onesie that can assess a tot’s gross motor skills and whether the kiddo has reached those all-important developmental milestones on time.
Colonoscopy vs. FIT: Acceptance and efficacy
Are people more likely to accept an “invitation” to be screened for colon cancer by colonoscopy or by a fecal test (FIT)? And for those who do get screened, does the type of test affect their chance of having cancer within 10 years?
A new study of over 57K people got the following answers to these questions. The subjects were randomly assigned - half received an invitation to get a colonoscopy, and the other half were invited to take two FIT tests over the course of a year.
About 8% of people declined the invitation to be screened at all.
Of those who were offered a colonoscopy and said they’d be willing to have one done, about 32% went through with it.
Of the folks who were offered the FIT tests and said they’d be willing to be screened, about 40% actually took the tests.
The folks who got screened by colonoscopy had a 0.22% chance of dying of colorectal cancer within 10 years.
For people who took the FIT tests, that risk was 0.24%.
COMMENTARY: One quibble: The subjects in this study were 50 to 69 years old and had no risk factors for colorectal cancer. Given that incidence of colon cancer is rising particularly in folks under age 50, it would have been better had that age group been included.
But for the folks in the age cohort the study examined, this looks like a “yes, and” situation to us. If you’re willing to get that colonoscopy, do it. But if you’re not, take a FIT test twice a year. And if offering FIT tests gets more people screened, so much the better.
The FDA has approved the first non-prescription, fully at-home self test for sexually transmitted infections. The test diagnoses chlamydia, gonorrhea, and trichomoniasis and requires a vaginal swab. It returns results in about 30 minutes.