In This Issue
How and why the CDC’s COVID test kit failed
Fragmentomics streamlines liquid biopsy
Diagnostic data could decrease maternal, infant mortality
New and Noteworthy
White House executive order on AI includes focus on health and safety
This week, the Biden-Harris administration issued an executive order that aims to install some safety guardrails for medical AI - as part of an overall effort to put some regulatory reins on AI’s runaway thoroughbred. (FDA has been trying, but as yet looks like it can’t keep up.)
The order requires HHS to establish a safety program that receives reports of “harms or unsafe health care practices involving AI” and then works to fix them. It also requires builders of foundation models that impact public safety or health to notify the government when they’re training such a model, and to share the results of safety tests. (Here’s the nitty-gritty on exactly how the agency is supposed to get this stuff done.)
Foundation models, sometimes called “general-purpose AI,” are big, self-trained things that have been trained on a wide range of data and can produce a bunch of different kinds of outputs. AI that’s intended for more narrow uses is often built on top of a foundation model, so anything that’s wrong with the foundation will also be wrong with whatever’s built on top of it. (The Ada Lovelace Institute has a good explainer of this complex topic.)
Commentary: We applaud the administration for starting this process. But clearly this is only the beginning of the beginning - much more to come. Most importantly, regulations need to be relevant and reasonably protective without squashing innovation.
How and why the CDC’s COVID test kit failed: Lack of experience, resources, oversight, and tracking
The office of the inspector general (OIG) for HHS has issued its report examining how and why the CDC’s initial COVID test failed. The short version of the answer is right there in the report’s unwieldy title, clearly aimed at “TL;DR” (Too Long; Don’t Read) folks: “CDC’s Internal Control Weaknesses Led To Its Initial COVID-19 Test Kit Failure, But CDC Ultimately Created A Working Test Kit.”
The details:
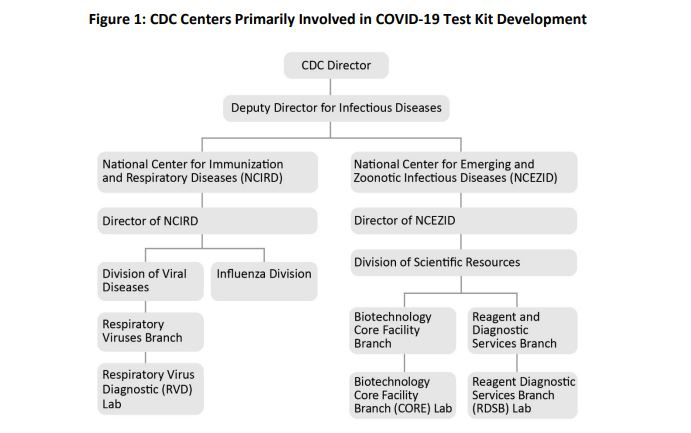
The lab responsible for making these test kits (the Respiratory Virus Diagnostics lab, or RVD) was a research lab, with “no policies and procedures for developing and manufacturing test kits.”
Test kits were sent out to public health labs before they underwent quality-control testing. (OIG also attributes the error to the lack of policies and procedures.)
RVD didn’t get the staff they needed to do the job properly, despite repeated requests (the word “pleaded” comes up in the report).
CDC didn’t have virus samples with which to validate the test, so RVD asked other labs, including outside manufacturers and CDC’s Biotechnology Core Facility Branch (CORE) lab, to make samples based on genetic sequences of the virus. When the manufacturers turned them down due to prior commitments, CORE ended up having to make both the virus samples and reagents for the tests in the same laboratory, something they would never normally do. Despite “strict workflow controls,” it’s possible that contamination happened at this stage.
RVD lacked oversight from both its division of CDC and, when the emergency got worse, from the agency as a whole.
CDC didn’t have a laboratory document control system or a laboratory quality management system (QMS).
CDC responded to the report by noting that it’s already solved - or is in the process of solving - many of these issues. It has developed a Laboratory Quality Plan, has “published documentation outlining laboratory functions during an emergency response,” and improved oversight of emergency response efforts. It’s working on implementing an electronic QMS.
Commentary: Three main thoughts here.
While we would have liked to see this report come out sooner, it’s great that OIG analyzed the situation thoroughly.
If you are in the lab industry (or almost any regulated industry), you will recognize that these errors are so obvious, it is frustrating to read them.
Why were the RVD pleas for help ignored? Did the administration in 2020 not believe the researchers? Did the administration believe that test creation was easy and therefore they really didn’t need help? We know how hard it was to create a novel vaccine for COVID, how tricky it is to target an antiviral to a new virus. But it is not simple to develop an accurate test either. Let’s stop taking diagnostics R&D for granted.
Food for Thought
A new approach to liquid biopsy - harnessing fragmentomics with AI
Cancer is a genomic disease: Mutations accumulate and DNA gets disorganized. Most liquid biopsy tests look for characteristic mutations, but that is an expensive and difficult process, requiring amplification of gene regions followed by costly deep sequencing to find single mutations hiding in a 3.2-billion-base sea of DNA.
A new lung-cancer test avoids the “needle in a haystack” problem by using “fragmentomics”: looking for a pattern of cell-free DNA in blood that’s indicative of cancer. It’s much easier data to collect (shallower sequencing), and uses AI to distinguish patterns typical of different cancer types. Early data on this test were published in Nature (2019); at that time, the test had 73% sensitivity and 98% specificity. A test rollout is to begin shortly for this now 99.7% negative predictive value diagnostic.
Commentary: Here is yet another arena where AI is allowing us to diagnose more with less - less invasive, less analysis, lower cost.
Diagnostic data from AI/ML-boosted tools could decrease maternal, infant mortality
Sadly, it is well documented that the US has the highest maternal mortality rates amongst industrialized countries. And as high as the overall rate is, the rate for black women is three times higher.
Looking at infant mortality rates, the US is also high compared to other developed nations (and our rate just rose by the largest amount in decades). Important to note, however, that infant mortality is defined differently and more broadly in the US than it is elsewhere, which may contribute to a somewhat elevated US statistic. Within the US, black women have a 1.5 times higher likelihood of preterm labor, a prime contributor to infant mortality.
Diagnostic data can help.
Data from wearables showed that deviations from normal sleep sleep activity patterns can predict risk of preterm birth.
AI-based algorithms can find outcome patterns by analyzing ICD-10 social determinants of health diagnosis codes along with medical records. Those patterns can help pinpoint women with a higher likelihood of adverse outcomes.
Quick Hits
The FDA has approved the first OTC test for the preliminary detection of fentanyl in urine - 16 days after the test was submitted to the agency for marketing approval. Test-takers are supposed to send samples to the manufacturer’s laboratory for confirmation.
The Traveler-Based Genomic Surveillance Program flagged a sample from Dulles International Airport as containing the Omicron variant BA.2.86 on August 17, 2023, less than five days after the novel variant was first sequenced in Denmark and Israel. The sample was traced to a passenger traveling from Japan - and the identification was made 17 days before BA.2.86 was first sequenced in that country.
EUA Update
The FDA issued one new 510(k) premarket notification, one new EUA, one amendment to existing EUAs, and one revocation in October. Data is available at TestingCommons.com
510(k) Premarket Notifications (1): Xpert® Xpress Cov-2 Plus
New EUAs (1):
COVID Molecular: (1) MAWD Laboratories
Amendments to Existing EUA’s (1):
COVID Antigen: (1) Maxim Biomedical, Inc.
Revocations (1): SARS-CoV-2 DUCoM-PDL Modified Tetracore Assay