COVID: Will the summer surge become the autumn acceleration and winter wave?
Volume 8, Issue 11 | August 23, 2023
In This Issue
The new variant: New Vaccines and Old Tests
WHO’s seven commandments for a world with COVID
CDC learnings from the failure of the original COVID test
COVID breath tests
New and Noteworthy
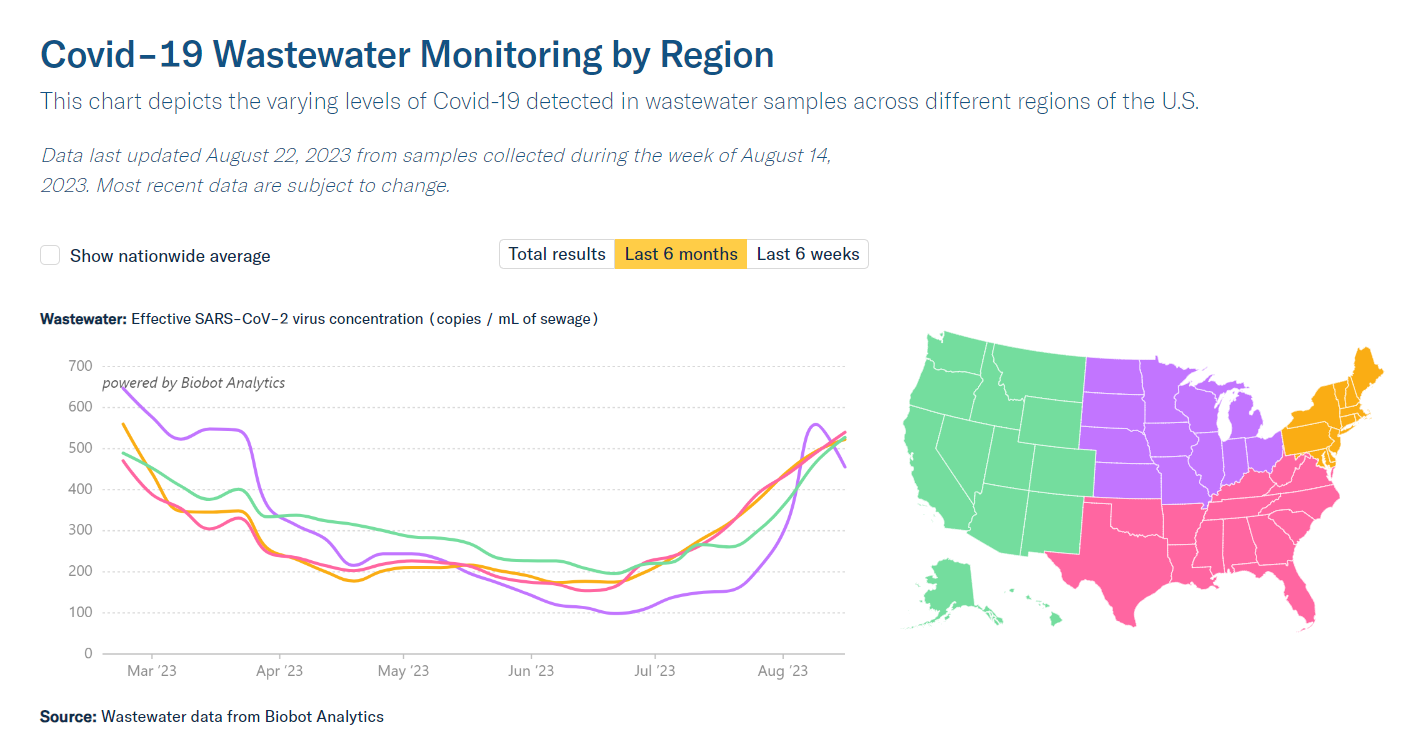
As has been widely reported, COVID levels have risen this summer all through the US. No one knows how many cases there are, because few reporting systems are still functional, but wastewater levels are “the highest they’ve been since last winter’s holiday surge,” according to the COVID-19 Data Dispatch. So, we are back to cover COVID this week.
New vaccines and the new variant
As we head toward winter in the northern hemisphere, our vaccine preparedness looks pretty good:
The 2023 quadrivalent influenza vaccine is expected to be a good fit for northern hemisphere variants.
For the first time, RSV vaccines are available to protect the most vulnerable: pregnant women (Pfizer Abrysvo), infants (Sanofi/AstraZeneca Beyfortus), and the over-60s (GSK Arexvy & Pfizer Abrysvo).
Updated COVID vaccines are expected to be available at the end of September (plus, 98% in the US have some pre-existing immunity from natural exposure and/or past vaccination). Given the current rise in prevalence, it would have been good to have them earlier, but it’s hard to imagine that uptake would have been high enough to make an appreciable difference.
When the FDA external expert group (VRBPAC) met in mid-June to plan COVID vaccine updates, XBB.1.5 (aka Kraken) represented 70% of sequences, but by the end of July that had dropped to 5%. The biggest concern right now is the new and potentially fast-growing BA.2.86 (aka Pirola), which shares most XBB.1.5 spike mutations and adds 30 more. Genetically speaking, it’s as different from XBB.1.5 as Omicron was from Delta, but it’s too early to predict whether its morbidity will be significantly different from XBB.1.5’s.
Old tests and the new variant
From a diagnostic point of view, Taqpath PCR’s S-gene target failure is the gift that keeps on giving. Each succeeding wave of COVID either has had or not had the Del69-70 deletion that causes it, as follows:
Alpha had the deletion → Delta didn’t → original Omicron had it → XBB.1.5 doesn’t → BA.2.86 does.
That gives us an inexpensive way to track which variant is causing any case that’s diagnosed by PCR, without having to sequence. (For a great overview of the BA.2.86 situation, see: Your Local Epidemiologist’s recent post; Nature has a good short article; Jesse Bloom Lab at Fred Hutch Cancer Center/HHMI has a detailed review)
Thinking about COVID at-home tests, there is every reason to believe that they will all be able to recognize the BA.2.86 variant. Why are we optimistic? Most of the differences between BA.2.86 and previous Omicron variants are in the spike protein, and the antigen tests don’t look for that. Antigen tests look for the N protein. BA.2.86 has only one “N” mutation (Q229K), that differs from prior BA.2 variants, and this will not change the N protein significantly.
Commentary: We believe that this summer surge will become an autumn acceleration; crossing our fingers that it won’t turn into a winter wave.
Seven commandments for a world with COVID: Thus spake WHO
The WHO released standing recommendations for COVID at the beginning of August, replacing temporary recommendations made when they rescinded the international public health emergency in May. The recommendations to the nations of the world fall into seven categories, here translated from government-speak:
Revise and actually use COVID plans and policies that adhere to the WHO COVID-19 Strategic Preparedness and Response Plan April 2023- April 2025.
Keep up surveillance for COVID and talk to each other about what you find.
Keep reporting COVID epidemiological data, including mortality, morbidity, sequencing (with metadata), and vaccine effectiveness.
Use WHO recommendations and your own national priorities (including “cost-benefit reviews”) to decide who should get a COVID vaccine, and make sure vaccine delivery fits into your health system. Oh and by the way, do your best to deal with vaccine misinformation.
Keep researching how to “prevent and control” COVID.
Use COVID treatments that we know work, and don’t forget to protect health care workers and caregivers.
Keep working towards equitable access to good diagnostics, vaccines, and treatments for COVID, both within your country and around the world.
Commentary: Sorry to say this, but we doubt that many nations will have the money, the resources, or the interest to implement these actions. We understand that the WHO needs to issue these and put pressure on governments to implement, but we would prioritize #2 - keep up some sort of surveillance and encourage nations to talk to each other, or at least to the WHO. That may be the best offense against the virus we have right now.
In February 2020, the CDC failed. It’s trying to learn from that.
It is undisputed that the very first COVID test developed at the CDC in February 2020 was inaccurate and couldn’t be implemented. Why did this happen? Well, failure has many owners. But the more important question today is how will the CDC avoid this happening again?
In 2021, CDC convened what it calls the Laboratory Workgroup to answer this critical question. The group issued a comprehensive and efficient (20 pages) report on what must change at the agency in order to improve testing operations. The major points:
Centralize leadership of lab operations in a single individual who reports to the CDC director
Centralize lab support operations, including quality control, and enhance focus on quality systems
Improve communications across the CDC
Separate the labs that conduct research from those who do clinical diagnostics work
Utilize external experts to help with future test scale-up
And critically: “The CDC should lead the standardization of health data collection associated with laboratory tests to improve future public health responses.” This should include obtaining national agreement regarding use of a minimal data set in an electronically transmissible format, which should be required to initiate testing in biological emergencies.
Commentary: It’s easy to say, “It’s great that the CDC convened a group of experts to look at their past mistakes.” It’s hard to ensure that the relevant people read the report and act on it. It’s even harder to get Congress to provide an adequate budget to implement the necessary changes. That said, CDC has already taken steps in the direction that the Laboratory Workgroup recommended, by establishing the new Center for Laboratory Systems and Response. It’s a start.
Food for Thought
Good early signs for two more COVID breath tests
Breath tests were always considered the holy grail for COVID detection. One breath test received an FDA EUA in April 2022 but there was little adoption. Two new tests have seen success recently, though neither is quite ready for prime time yet.
One arrived in the news in late July, with a study published in ACS Sensors. Though the study was teeny-tiny (eight subjects, two COVID-negative and six COVID-positive per PCR), it was right on the money every time, after two breaths and 60 seconds. The other, which received Phase I funding from NIH’s RADx program at the beginning of August, is mask-based technology that collects exhaled breath condensate while the patient breathes normally - a potential boon for people with disabilities.
Quick Hits
A study in the September 2023 issue of Emerging Infectious Diseases found that people who tested positive on home tests were 29% less likely to isolate than people who got a positive result on a test from a health care provider. In addition, home testers who isolated did so for two fewer days on average than folks who tested with a provider.