ALSO IN THIS ISSUE
FDA updates COVID test development guidance
Constructive criticism by HHS on FDA’s early pandemic response
Decreased worldwide testing hampers WHO’s ability to track new variants
Plus: Tracking a mutated virus to one set of toilets
New and Noteworthy
Who goes to the front of the line? New FDA test development guidance
The FDA updated its guidance for COVID test developers this week, mostly by saying, “Remember what we told you last year? Well, it still applies.” But there were a few changes.
The agency will now prioritize review of EUA requests and supplemental EUA requests from developers that have had their EUAs authorized in the past. (Shepherding the newbies through the process takes too much time and resources.) Among those developers, they’ll prioritize diagnostic tests that are likely to be game-changers and/or those that “fulfill an unmet need,” in particular “diagnosing infection with a new variant or subvariant.”
If your test comes from or is financed by the feds (think BARDA and NIH’s RADx), you’re also in luck - you get to go to the front of the line, too. For everyone else, FDA very politely recommends using “traditional pre-market pathways.”
Yes, it was a bit of a mess - but we know you were trying. HHS reports on FDA’s early pandemic response
The HHS Office of the Inspector General released a report last week on the FDA’s early (January - May 2020) response to the pandemic. By comparison with the somewhat scathing headlines we’ve seen about it, the document itself reads like a gentle rebuke from parents telling a kid that yes, you made a few mistakes on that last assignment, but we know you were trying, and you can do better next time.
So what did the FDA do wrong? They didn’t follow up with public-health labs well enough to realize that the first available COVID test, from the CDC, wasn’t working. They let some crummy tests onto the market because they decreased the levels of evidence required for an EUA. HHS does acknowledge, however, that tests needed to get to market asap, and available samples were limited at the time. (Important to note: Virtually all of these questionable tests were serology / antibody tests and the FDA recalled most of them later in the pandemic.) They opened the EUA process to anyone and everyone and discovered that unless you’d done the whole EUA thing before (see article above), the process was too complicated for many submitters.
The FDA agreed with everything HHS had to say, including all of its recommendations on how to improve things.
Commentary: As the report notes, the FDA should put better systems in place for communicating with labs and diagnostic manufacturers and needs to improve and streamline guidance for EUA submissions. The FDA did and does continue to host regular Testing Town Halls which are important meetings but agree that more communications would have been useful, especially in the pandemic’s first year and when regulatory changes were made. However, fundamentally, some of the things that went wrong were and remain out of the agency’s control.
Looking into the very near future, we would add that the FDA needs to ensure that their diagnostics teams are appropriately staffed. We know that this report is looking back - but we need to look forward. Fast response times will be particularly critical in the coming months when the FDA will need to respond to continuing EUA applications (likely more complex ones with new technologies including those from RADx - see above) AND the tsunami of 510k applications that have already started and will continue to grow through the end of 2022 and into 2023.
We can’t find what we don’t look for - including new COVID variants
Last week, the WHO’s COVID Technical Lead, Maria Van Kerkhove, reported that with decreased testing in so many nations, her organization is having difficulty keeping track of new COVID variants. The testing cutbacks are happening because of reduced case numbers (the good news), but the nations in question have also cut back on surveillance (the very bad news). WHO is watching roughly 200 sub-variants of Omicron at the moment, focusing particularly on these three (percentages apply to the US as of 9/24/22).
BA.4.6 (11.9%)
BF.7 (2.3%)
BA.2.75 (1.4%)
Commentary: Testing is the only way to predict a surge and then slow it down! (No apologies for the exclamation point.)
Food for Thought
Cancer screening by blood test: Are “liquid biopsies” the future?
The past 20 years have seen growing momentum in the quest for a “liquid biopsy”: a simple blood test that can measure and sequence cell-free cancer DNA. Several companies (Grail, Freenome, Guardant) are attempting to reach this concept’s ultimate goal: a blood test that detects most cancer types and their organs of origin.
Grail recently provided an update on the clinical trial for their pan-cancer blood-test product, Galleri. They reported that 38% of Galleri positives were confirmed to have cancer (positive predictive value of 38%). These positives, Grail says, are individuals who might not have been otherwise diagnosed (although we cannot be certain of this - see this 2021 paper for a nuanced discussion). But let’s not forget - the implied false positive rate was 62%. All of the people in that group would have undergone further investigative procedures that would ultimately have been unnecessary.
Modern cancer genomics began with single-drug companion diagnostics to find whether this drug would work for this patient. As the number of available therapies has grown, broader panels of genomic tests have been designed to help oncologists find the best therapies for their patients (e.g., Foundation Medicine’s FoundationOneLiquid CDx, which examines 324 genes for treatable mutations).
However, screening for cancer is, by its nature, much more challenging than therapy selection. All tests generate some rate of false positives, and if the vast majority being tested are negative, a small percentage of false positives can become a huge number of people subjected to unnecessary further procedures and a very large degree of stress. Example: On average, 0.6% of the US population gets cancer each year. Thus, if you’re screening the whole population, even an exceptionally good test - one with a 0.5% false positive rate - will generate near-equal numbers of false and true positive results.
Commentary: The way to mitigate this problem is to focus screening on sub-populations with higher incidence rates (e.g., older, at-risk, and/or symptomatic patients). This is one reason that many companies focus test development based on an individual cancer type, using more markers to screen for higher-incidence cancers in higher-incidence sub-populations. That’s especially critical when existing tests are more invasive, onerous, and/or expensive (e.g., surgery or imaging).
The underlying need for better cancer screening is clear. We believe that liquid biopsy has great potential for early diagnosis, which would be especially powerful for cancers with few or non-specific early symptoms. Is liquid biopsy ready for wide adoption? A lot of money is pouring into the field, but with much still to be done, the data is not yet clear.
EUA Update
The FDA issued 12 amendments to existing EUAs and one revocation in the last four weeks (since the August 31st Newsletter)
Amendments to Existing EUA’s (12):
Molecular (9): University of Louisville | Thermo Fisher Taqpath (2) | INNO Diagnostics | Uh-Oh Labs | PerkinElmer | Rheonix | Predicine | LumiraDx | Quidel Lyra
Antigen (2): Xiamen Boson | ACON FlowFlex
Serology (1): LumiraDx
Safety Communications (1):
Revocations (1): Talis Biomedical
Monthly Capacity: Current EUAs
Small reduction in capacity during September. The predominant approach now is “wait and see”. There are three major factors in consideration:
#1 Who will win the Federal RFP for the next order of OTC rapid antigen tests? The Administration has emphasized a strong preference for US manufacturers.
#2 What is the likelihood of a fall / winter surge as we have seen in the last two years?
#3 Will the FDA authorize or approve COVID / Flu combination test(s) for self / home use?
Quick Hits
Before meeting President Biden, reporters must still take a same-day PCR test. Our take: the emergency phase of the pandemic can be over without COVID being over. In fact, this is exactly what post-pandemic life should look like: People taking sensible steps to avoid getting ill. People who are at higher risk (and the POTUS is, since he’s over 65) need to take more steps - in his case, that means requiring a same-day PCR rather than a same-day antigen test, which might not pick up an early infection.
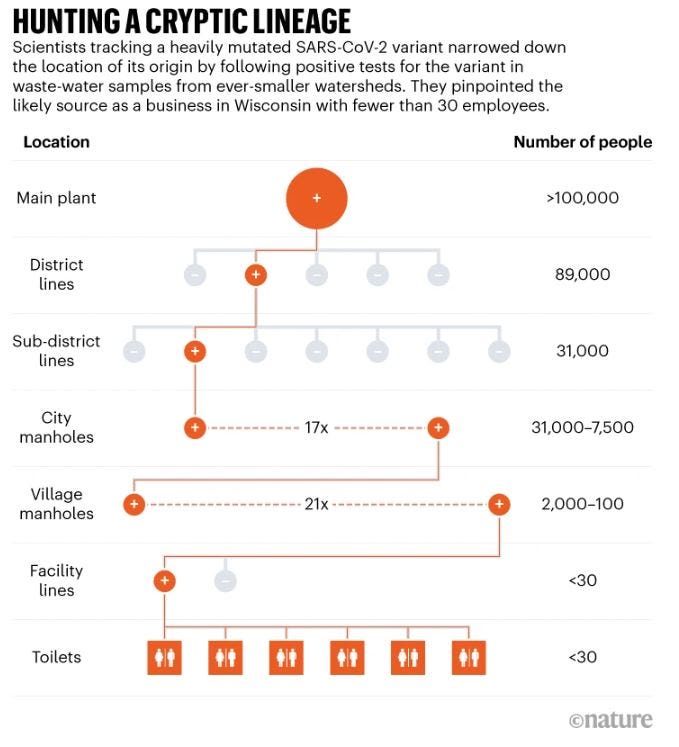
Researchers in Wisconsin used wastewater to track a significantly mutated SARS-CoV-2 variant to a single business with fewer than 30 employees. They’re now working to identify the source, which is likely to be a one person who’s been infected for more than eight months - a classic example of how new variants arise.
Printed in Nature: 26 September 2022