In This Issue
Solving the puzzle of early Parkinson’s Dx
Real-time SARS-CoV-2 air monitoring is (almost) here
For COVID antigen tests, serial testing is required. Period.
Follow ups: GISAID; patient knowledge of test results
New and Noteworthy
A new era for Alzheimer’s Dx and Rx
With the full FDA approval of Lecanemab for slowing the progression of Alzheimer’s disease (AD) in patients with mild cognitive impairment, the interest in AD diagnostics has soared. The current standard of care for diagnosis still begins with pencil-and-paper exams to measure changes in degree of “dementia” (e.g., memory and cognitive and executive function) but has rapidly incorporated imaging (MRI and PET). Sample-based tests are moving away from using cerebrospinal fluid, turning instead to blood biomarker tests that measure levels and types of tau and amyloid beta. And a new, non-invasive potential entry to the market combines an online cognitive test with AI-based analysis of speech patterns.
Commentary: We expect significant increases in the number - and hopefully the sophistication - of diagnostic tools to come. We also expect at least in the short term to see multiple diagnostic tools using different technologies - either independently or in conjunction with each other. Until we understand far more about the complexity of Alzheimer’s, its highly variable nature, and its shared pathology with other neurodegenerative diseases (especially in the early stages), we will likely not see a one-size-fits-all test anytime soon.
A solution to the puzzle of early Parkinson’s diagnosis?
Degenerative neurological disorders have always been frustratingly difficult to definitively detect. By the time diagnosis finally happens, the brain typically has incurred so much damage that halting further deterioration is impossible - making research on diagnosis and treatment impossible, as well. Parkinson’s is a classic example. On average, pre-Parkinson’s (aka prodromal Parkinson’s) has been present for more than four years by the time individuals are diagnosed with the disease; by that time, 70% of the relevant neurons in the brain will have been destroyed.
A report in Nature Medicine details a potential solution for Parkinson’s, at least: AI can analyze accelerometer data to predict the disease up to seven years before full diagnosis. Inexpensive accelerometers are widely available thanks to their gaming and smartphone applications, and can detect a wide range of movement parameters. Parkinson's patients exhibit movement initiation hesitancy, manifesting as reduced acceleration (by as much as a third) in prodromal patients compared to healthy controls. Accelerometers can measure this hesitancy 80 times per second (80Hz) in three dimensions.
But the data sets that result are enormous - 150 million data points per patient per week - and so heterogeneous that human observation cannot detect the early patterns that represent disease. That’s where machine learning AI comes into its own, permitting identification of this highly predictive feature four to five years before the disease becomes otherwise apparent.
Closing in on the holy grail of COVID surveillance: Detecting virus in the air
A Nature Communications paper last week described a successful proof-of-concept test for an air monitor that can detect the SARS-CoV-2 virus in real time (five minutes from sampling to result). Air-sampling devices for this purpose already exist, but they require the sample to be sent to a lab for PCR testing, limiting their utility.
In laboratory tests, the monitor’s biosensor demonstrated 77 - 83% sensitivity and was able to detect a range of SARS-CoV-2 variants. (With multiplexing, it could eventually be used to detect other pathogens, as well.) In the tiny real-world section of the test (two asymptomatic COVID patients, seven air samples from their bedrooms, and three air samples from a virus-free room), its sampling system batted 1000, collecting enough virus to test positive on PCR every time. (Of note: The positive air samples had high Ct values (32.7 - 34.9), indicating that the system would work even when infected people in the room don’t shed much virus.)
While these results are exciting, there’s one big problem. This sucker is loud, clocking in at 75 - 80 decibels (dB) - louder than the average vacuum cleaner. The authors are working to get that level down below 65dB - air-conditioner territory - in hopes that it can one day be deployed in places like schools, hospitals, and long-term-care facilities.
Food for Thought
The final (?) word on COVID antigen tests: You gotta test at least twice.
We and others have commented many times that PCR/antigen test comparisons need to ignore the long tail of post-infection PCR positives in patients who are no longer a transmission risk. Annals of Internal Medicine’s prospective study did that, looking at the performance of the two test types at the beginning of infections - when PCR tests have a theoretical one- to two-day advantage over antigen tests, offset by the typical one to two days it takes to get PCR results.
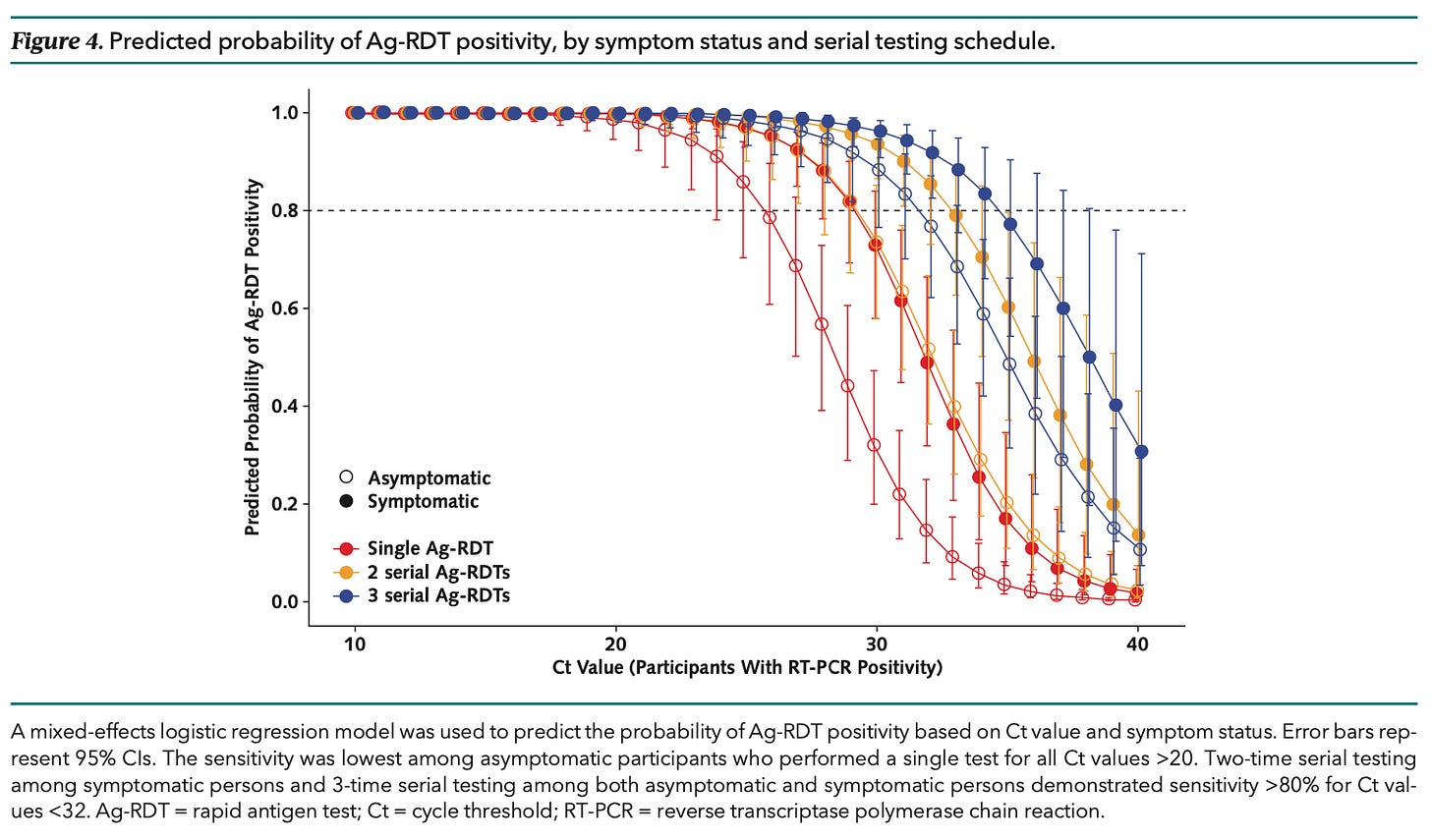
Bottom line: Antigen tests were 99.6% specific, but sensitivity on the first day was only 59.6% for patients with symptoms and just 11.7% for those who were asymptomatic. Sensitivity in the latter group went up to 50.7% with a second test 48 hours later, and got to 74.6% with a third test 48 hours after that.
Commentary: Transmission is at least in part a function of viral load, and asymptomatic patients both have lower viral loads and expel less virus into the environment at any given load. While that distinction has become less important as the virus has mutated to become more easily transmitted, it still matters at least a bit. A chart from this paper (shown here) illustrates how the probability of antigen test positivity varies, depending on the amount of virus in a sample: “Two-time serial testing among symptomatic persons and three-time serial testing among [both groups] demonstrated sensitivity >80% for Ct values < 32.” The question is, now that the virus is so exquisitely contagious, is 32 a reasonable cutoff for transmissibility?
Patients want access to test results ASAP.
Continuing on our recent discussion about patient knowledge of their own test results before their clinicians, we noted that the 21st Century Cures Act requires that patients have access to their medical results immediately. In a study of more than 8,000 people, 96% of them preferred immediate access to their health data even if it was before their medical team reviewed the results. The study did note however, increased worry occurred in 16.5% of those with abnormal results (versus 5% with normal results).
Why GISAID? Timing was everything.
Back in May, we discussed the shenanigans at GISAID, the most popular open-access repository for COVID genome sequences. (A big hat tip to the reporters at Science, who investigated and broke the story.)
If you’re still wondering how GISAID became the go-to data bank of the pandemic, the New England Journal of Medicine gave an answer this week with a perspective on the available data banks and how their differing rules and capabilities determined GISAID’s dominance. A companion perspective discusses public health data needs in greater detail.
Quick Hits
Researchers have created an ELISA test that can detect antibodies to SARS-CoV-2 in any animal species. Unlike other such tests, which typically look for species-specific antibodies to membrane-bound viral proteins, this test targets antibodies to the virus’s N-protein, which are conserved across species.
A Financial Times opinion last week questions the public health value of “general preparedness” for a pandemic. Instead, the author recommends a highly flexible testing-oriented strategy - the capability to roll out tests very very quickly, so that transmitting individuals can be swiftly isolated.
EUA Update
The FDA issued one new 510(k) premarket notification, three new EUAs, 16 amendments to existing EUAs, and four revocations in June. Data is available at TestingCommons.com
510(k) Premarket Notifications (1): Roche cobas SARS-CoV-2 Qualitative for use on the cobas 5800/6800/8800 Systems
New EUAs (3):
COVID Molecular (3): Acutis Diagnostics SARS-CoV-2 Acutis Multiplex Assay |Michigan State Laboratories In-Dx SARS-CoV-2 RT-LAMP Assay | Discover Labs COVID-19 Assay
Amendments to Existing EUA’s (16):
COVID Molecular: 9
COVID Collection Kits: 0
COVID Antigen: 6
Respiratory multiplex (COVID and Flu): 1
Revocations (4): Verily Life Sciences | BD Veritor At-Home COVID-19 Test | Biosearch Technologies SARS-CoV-2 Real-Time and End-Point RT-PCR Test | Biosearch Technologies SARS-CoV-2 ultra-high-throughput End-Point RT-PCR Test