ALSO IN THIS ISSUE
Will these two pTaus be the key to early Alzheimer’s dx?
AI decision support might help diagnose CRC earlier
Bird Flu Update: Asymptomatic infections in people
At-home test for aggressive prostate cancer could be on the way
A urine test for aggressive prostate cancer could one day be used as an at-home test, thanks to the results from a recent validation trial. The test looks at 18 different genes linked to high-grade prostate cancer - the kind more likely to cause harm.

A previous study showed that the test was effective, but in that trial, urine samples were obtained after a digital rectal exam (DRE). This study found that the test worked well even without a DRE. When combined with clinical signs and prostate volume, it achieved about 77% accuracy - a lot better than the good old prostate-specific antigen test, at 57%.
Will these two pTaus be the key to early Alzheimer’s diagnosis?
Early Alzheimer’s disease diagnosis has a pair of big challenges: finding biomarkers for disease before brain degeneration has become too advanced to treat, and distinguishing Alzheimer’s from other quite different brain disorders. This week Nature Medicine published further research into early-stage tau protein biomarkers for Alzheimer’s, one potentially fruitful line of inquiry.
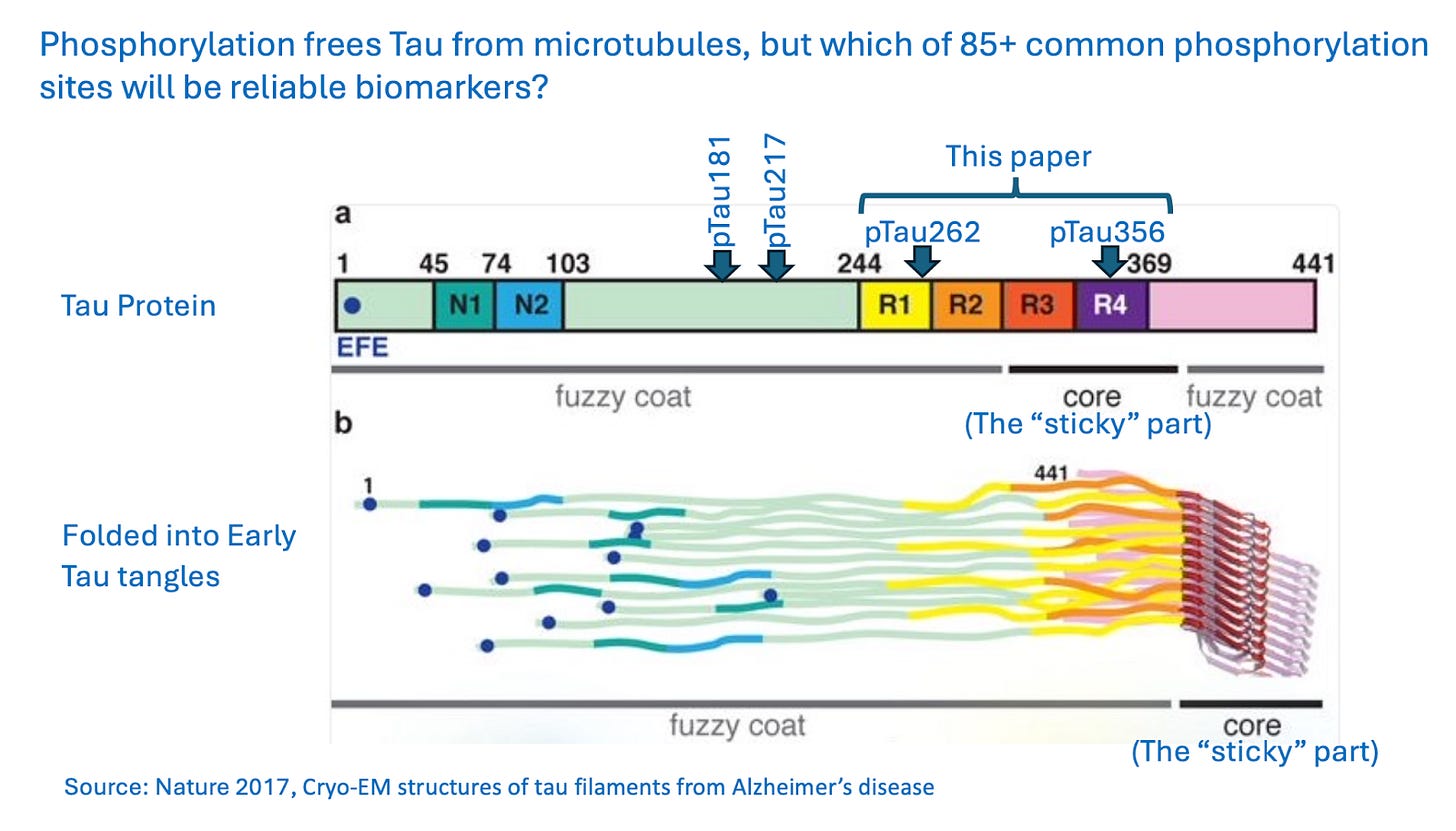
We have previously discussed the effectiveness of blood tests for phosphorylated tau proteins (pTaus181, 205, 212, 217, 231). The researchers here believe that these sites may miss true disease because those parts of the tau protein are in the “fuzzy coat” of the protein, not the “sticky” core where tangles first form. (They do acknowledge that fuzzy-coat biomarkers are detectable early in Alzheimer’s progression.)
Instead, the test described in this paper looks for pTau262 and pTau356, which are closer to that “sticky” part. The researchers argue that those sites are more reliable markers for true disease – when tau is not just present, but is beginning to tangle. (The test uses cerebrospinal fluid, not blood, and the paper does not directly compare pTau262 and pTau356 with other types of pTau.)
COMMENTARY: For 20 years, Alzheimer’s research focused on removing amyloid beta plaques. When plaque-destroying therapies turned out to be disappointing, the focus turned to tau tangles and their raw material: excess phosphorylated tau.
But even now, we are by no means sure we are finding markers of the earliest stages of disease, because we are not confident that we really know the full Alzheimer’s cascade from cause to effect. (If you want to dig further into how Alzheimer’s begins and proceeds, this 2022 paper shows that 90% of all brain functions are going wrong by the time symptoms are apparent, and this 2025 paper suggests that defective transport between the neuron nucleus and its cytoplasm is what kicks off the whole cascade.)
We desperately need early-stage Alzheimer’s biomarkers. According to a 2024 paper, by the time patients receive a formal diagnosis, they’ve typically shown signs of cognitive decline for about six years, their hippocampi have been shrinking for about eight years, they’ve had elevated pTau for about 11 years, and have had amyloid beta in their brains for roughly 18 years.
This pTau262/356 paper increases our knowledge of the tau-tangling process, but which of the various pTau biomarkers will turn out to have the highest clinical utility is unknowable at present. The great hope is that some combination of these less-invasive biomarkers will eventually point the way to therapies that can be used early enough that they actually work.
Bird Flu Update:
Wild bird strain of virus found in cattle and passed to a human
Asymptomatic infections confirmed in people
The outbreak by the numbers - so far
The D1.1 strain of bird flu, which is the strain affecting wild birds, has been found in cattle for the first time. Soon after being detected in a dairy herd in Nevada, it was also confirmed in a dairy worker at the affected farm. This is the first time a person has gotten this strain of the disease from cows. It has been passed from birds to people in the past and in some cases has caused severe illness as well as one death in the US. The infected dairy worker had mild eye symptoms and has recovered.
The CDC has confirmed that people can be infected with H5N1 without having symptoms. The news comes from a study of 150 veterinarians who work with cattle. Three of them had antibodies to the virus, even though none reported exposure to infected animals.
As the outbreaks continue across the US, here’s where we are:
Cattle: 968 confirmed cases in 16 states. California hardest-hit, with more than 75% of their dairy herds infected (745 cases).
Poultry: According to CIDRAP, “a record 157.7 million birds” affected in all 50 states and Puerto Rico. Ohio hardest-hit in terms of number of birds, at nearly 21 million.
Domestic cats: 88 confirmed cases in 17 states. The H5N1 mortality rate in domestic cats is estimated at 67%.
People: 69 confirmed cases in 11 states. One death.
AI tool doesn’t always find CRC, but when it does, it’s early
Primary-care physicians are expected to do an awful lot in the short periods of time they have with their patients. Along with mental-health evaluations and dealing with acute physical complaints (among many other things), they also are supposed to keep an eye out for signs of cancer. It’s a lot.
At this year’s ASCO Gastrointestinal Cancers Symposium, researchers presented a poster evaluating an AI-based decision-support tool for primary-care clinicians. The question: How good was it at spotting the signs of colorectal cancer (CRC) in electronic medical record (EMR) data?
The tool was let loose on historical records from 900,000 patients on the Mayo Data Platform, of which 7,000 were subsequently diagnosed with CRC. It demonstrated sensitivity of 94% and pretty weak specificity of 20%. But strikingly, when it did detect cancer (29% of the time), it made the diagnosis up to five years before that diagnosis appeared in the EMR.
COMMENTARY: This particular trial was directed at CRC diagnosis, but the tool itself seeks out elevated risk across all cancer types. (Here’s a 2024 poster that describes the tool in more detail.) Given that the average primary-care physician sees about eight cancer cases a year, decision support is clearly needed.
AI in the background is one way to reduce time demands on clinicians, but false alerts from even an “in the background” system can be very costly. Trials are underway in the UK to further evaluate this particular tool’s effectiveness more broadly. While the presence of an all-condition, all-seeing, all-remembering AI wingman for primary care is an exciting prospect, we are not there yet.
Around this time last year, we reported that the FDA had approved “the first genetic test for possible increased propensity to develop opioid use disorder (OUD).” A recent case-control study published in JAMA Network Open cast doubts on the utility of the test. It found that the 15 genetic variants named in the test as likely predictors of opioid-use disorder “collectively accounted for 0.4% of the variation in OUD risk.” An AI model based on those 15 variants got the diagnosis right only 53% of the time.